Research
Research Overview

We provide chemical solutions (catalytic & adsorption materials/processes) for the sustainable growth of human society.
Development of renewable energy sources and environmentally benign chemical processes are essential for sustainable development of human society. With this respect, our primary research goal is to develop new advanced catalyst materials and catalytic processes that can contribute to the aforementioned issues, especially by using cutting edge knowledge on nanotechnology.
CO2 Capture and Utilization
Our research advances CO₂ capture and utilization technologies to address climate change by transforming carbon emissions into valuable products like fuels and specialty chemicals. In CO₂ capture, we’ve developed high-selectivity solid adsorbents with proven efficiency at pilot scale. For CO₂ utilization, our focus lies in industrially viable catalytic processes, such as methane dry reforming to produce syngas, which can be converted into valuable hydrocarbons. Through collaboration with industry, academia, and government, we aim to drive a sustainable circular carbon economy.
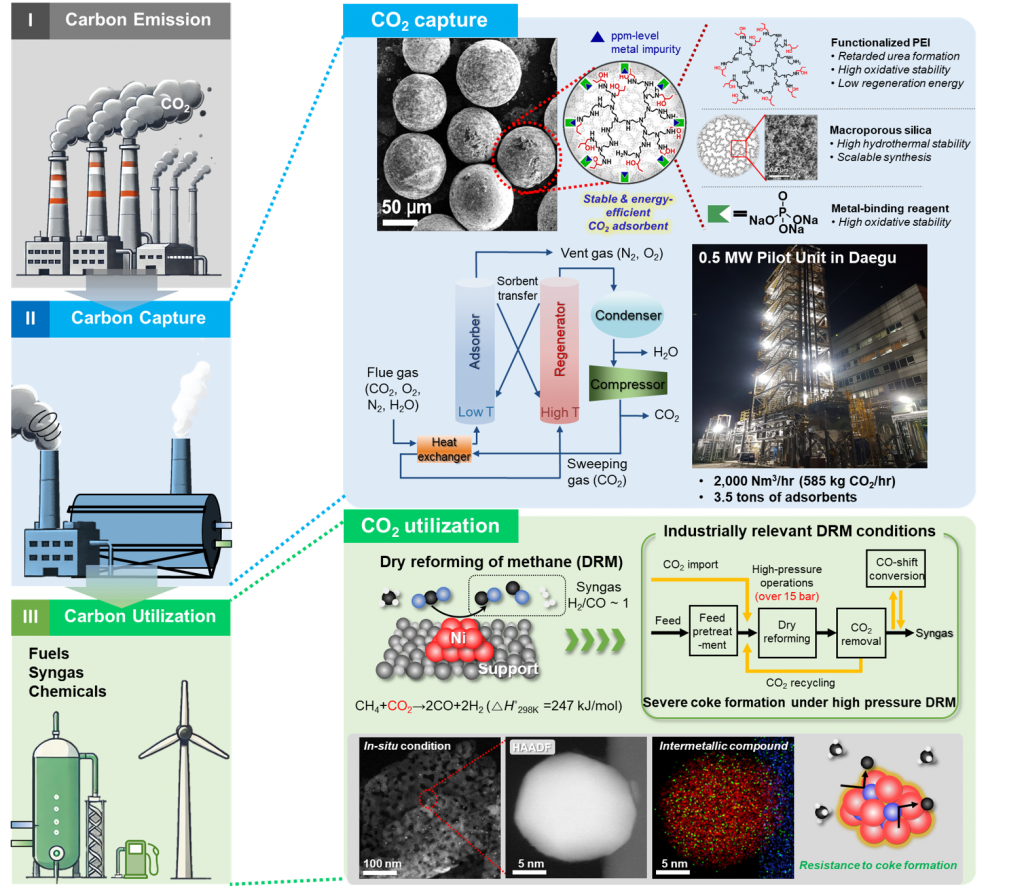
[1] “An Ethylenediamine‐Grafted Y Zeolite: a Highly Regenerable Carbon Dioxide Adsorbent via Temperature Swing Adsorption without Urea Formation”
Energy & Environmental Science, 9, 1803-1811 (2016) – Link
[2] “Epoxide-Functionalization of Polyethyleneimine for Synthesis of Stable Carbon Dioxide Adsorbent in Temperature Swing Adsorption”
Nature Communications, 7, 12640 (2016) – Link
[3] “Oxidation-stable amine-containing adsorbents for carbon dioxide capture”
Nature Communications, 9, 726 (2018) – Link
[4] “Design of Amine-Containing Nanoporous Materials for Postcombustion CO2 Capture form Engineering Perspectives”
Accounts of Chemical Research, 56, 2887-2897 (2023) – Link
Advanced Catalytic Production of NH3
We are advancing catalytic processes for green ammonia (NH3) production, addressing the growing demand for sustainable, carbon-free energy carriers. With ammonia increasingly gaining attention as a carbon-free hydrogen carrier, our innovations can contribute to the development of a cleaner, hydrogen-based energy infrastructure. Our focus is on developing catalysts that enable NH3 synthesis under markedly lower pressure and temperature conditions, making the process more energy-efficient and economically viable. This breakthrough is particularly promising for coupling with electrolytic hydrogen production, where the synergy between green hydrogen and N2 can significantly reduce carbon emissions.
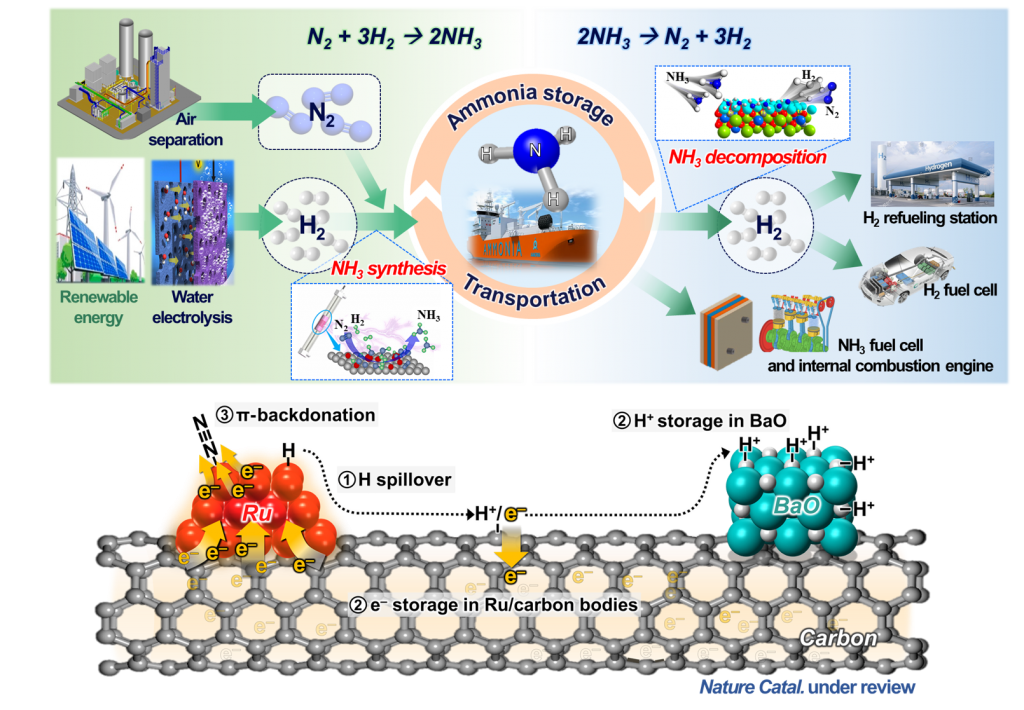
[1] “Splitting of Hydrogen Atoms into Proton-Electron Pairs at BaO-Ru Interfaces for Promoting Ammonia Synthesis under Mild Condition”
Journal of the American Chemical Society, 145, 11364-11374 (2023) – Link
[2] Nature Catalysis (Under review)
Biomass Conversion into Renewable Fuels and Chemicals
We are developing catalytic processes for the conversion of biomass into renewable fuels and chemicals, addressing the urgent need for sustainable energy solutions. Our research specifically focuses on the catalytic hydroupgrading of triglycerides, including vegetable oil, animal fat, and used cooking oil, to produce green diesel, sustainable aviation fuel (SAF), and high-value lubricant oil. By developing catalysts that enhance reaction efficiency and selectivity, we aim to create more energy-efficient pathways for converting biomass into valuable products. This innovation plays a crucial role in reducing carbon emissions and contributing to a more sustainable energy infrastructure, supporting the transition to low-carbon transportation and industry.
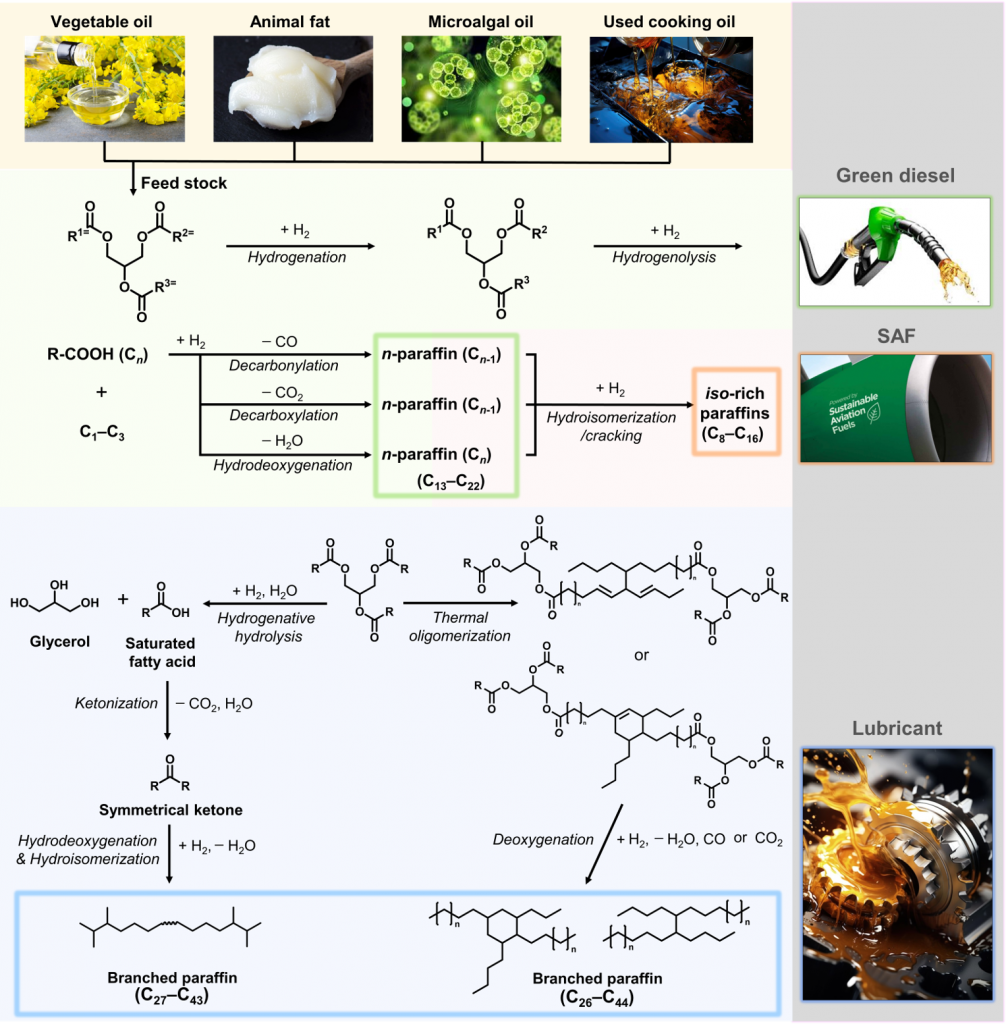
[1] “Maximizing Biojet Fuel Production from Triglyceride: Importance of the Hydrocracking Catalyst and Separate Deoxygenation/Hydrocracking Steps”
ACS Catalysis, 7, 6256-6267 (2017) – Link
[2] “Effects of Fatty Acid Structures on Ketonization Selectivity and Catalyst Deactivation”
ACS Sustainable Chemistry & Engineering, 6, 13035-13044 (2018) – Link
[3] “Effects of Fatty Acid Compositions on Heavy Oligomer Formation and Catalyst Deactivation during Deoxygenation of Triglycerides”
ACS Sustainable Chemistry & Engineering, 6, 17168-17177 (2018) – Link
[4] “Single-Step Hydroconversion of Triglycerides into Biojet Fuel Using CO-Tolerant PtRe Catalyst Supported on USY”
Journal of Catalysis, 379, 180-190 (2019) – Link
[5] “Coproduction of Value-Added Lube Base Oil and Green Diesel from Natural Triglycerides via a Simple Two-Step Process”
Industral & Engineering Chemistry Research, 59, 8946-8954 (2020) – Link
[6] “Importance of Pore Size and Lewis Acidity of Pt/Al2O3 for Mitigating Mass Transfer Limitation and Catalyst Fouling in Triglyceride Deoxygenation”
Chemical Engineering Journal, 439, 135530 (2022) – Link
[7] “Catalytic Conversion of Triglycerides into Diesel, Jet Fuel, and Lube Base Oil”
Chineses Journal of Catalysis, 58, 15-24 (2024) – Link
Plastic Chemical Recycling
We are developing catalytic processes for plastic chemical recycling, focusing on practical and industrially relevant approaches to tackle plastic waste. Our research is particularly focused on pyrolysis-based chemical recycling, which has gained significant industrial attention due to its ability to bypass complex plastic sorting processes. However, pyrolysis oil derived from waste plastics contains various impurities, such as Cl compounds from poly(vinyl chloride) decomposition, which can cause reactor corrosion and catalyst deactivation. We have developed a novel catalyst that exhibits exceptional catalytic activity and stability in dechlorination. This breakthrough enables efficient Cl removal with minimal use of precious metals like Pt, making the process both cost-effective and stable, contributing to scalable, sustainable plastic recycling. We are also working to develop more energy- and cost-efficient catalytic upgrading methods for plastic pyrolysis oils as well as for plastics themselves.
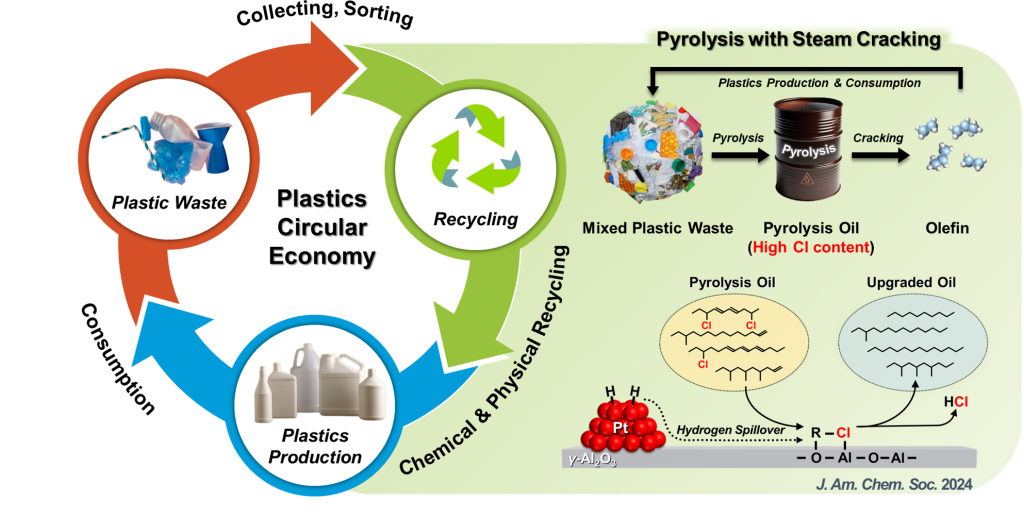
[1] “Catalytic Synergy between Lewis Acidic Alumina and Pt in Hydrodechlorination for Plastic Chemical Recycling”
Journal of the American Chemical Society, 146, 23881-23890 (2024) – Link
Energy-Efficient and Greener Catalytic Processes
We are developing advanced catalytic processes to create existing chemical products with significantly reduced energy consumption and environmental impact. By improving catalytic efficiency, we aim to minimize the energy requirements for key industrial reactions, which can lead to substantial reductions in greenhouse gas emissions and resource use. Our research emphasizes the importance of understanding catalytic mechanisms at a fundamental level, combining rigorous spectroscopic analysis with theoretical tools to unravel the complex interactions at play. This comprehensive approach allows us to design more efficient and sustainable catalysts, playing a vital role in reducing the environmental burden of industrial processes while maintaining high product yields and quality. In particular, we have a special interest in alleviating the mass transfer limitations in zeolite solid acid catalysts and minimizing the use of noble metals in catalyst design while enhancing catalytic activity, selectivity, and stability against coke formation and sintering. Target reactions include industrially important acid-catalyzed reactions, such as isomerization, cracking, and aromatization, as well as hydro/dehydrogenations, including selective acetylene hydrogenation and propane dehydrogenation.
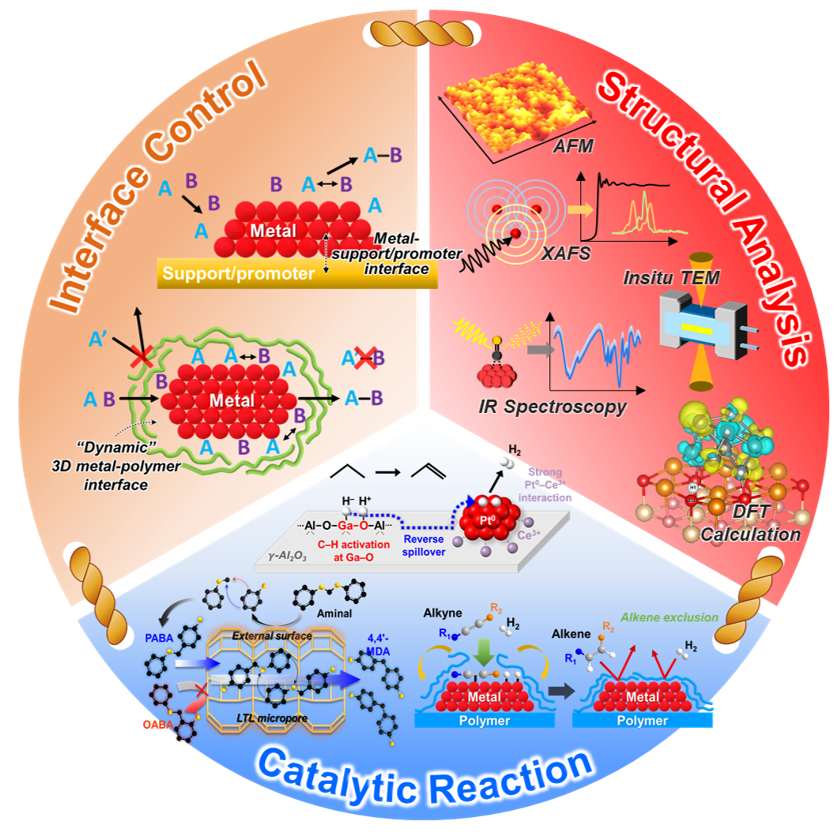
[1] “Dynamic Metal-Polymer Interaction for the Design of Chemoselective and Long-Lived Hydrogenation Catalysts”
Science Advances, 6, eabb7369 (2020) – Link
[2] “Tailoring Dynamic Metal-Polymer Interaction to Improve Catalyst Selectivity and Longevity in Hydrogenation”
Angewandte Chemie International Edition, 60, 12482-12489 (2021) – Link
[3] “Catalytic Interplay of Ga, Pt, and Ce on the Alumina Surface Enabling High Activity, Selectivity, and Stability in Propane Dehydrogenation”
ACS Catalysis, 11, 10767-10777 (2021) – Link
[4] “Hierarchical BEA Zeolite with Trimodal Micro-/Meso-/Macroporosity as a Selective and Long-Lived Catalyst for Isobutane/2-Butene Alkylation”
ACS Catalysis, 12, 4067-4077 (2022) – Link
[5] “Hierarchical LTL Zeolite as an Efficient and Sustainable Solid Acid Catalyst for Replacing HCl in the Production of Polyurethane Intermediates”
Angewandte Chemie International Edition, 62, e202304244 (2023) – Link
[6] “Cooperative Interplay of Micropores/Mesopores of Hierarchical Zeolite in Chemical Production”
ACS Catalysis, 14, 2031-2048 (2024) – Link
[7] “Synergistic Combination of Inorganic and Organic Promoters on Palladium Catalysts for Effective Acetylene Partial Hydrogenation”
ACS Catalysis, 14, 2938-2948 (2024) – Link
Exploring Cutting-Edge Material Science
Exploring cutting-edge material science, we have a fundamental interest in designing novel porous materials at the atomic scale for various applications in separation, catalysis, and energy storage. Our research encompasses a diverse range of materials, including zeolites (microporous aluminosilicates), inorganic oxides, carbons, functional polymers, and their composites. By manipulating material properties at the atomic level, we aim to enhance performance and efficiency across multiple applications, contributing to advancements in sustainable technologies. This innovative approach allows us to create tailored materials that can meet the specific demands of modern industrial processes while addressing critical challenges in energy efficiency and resource utilization.
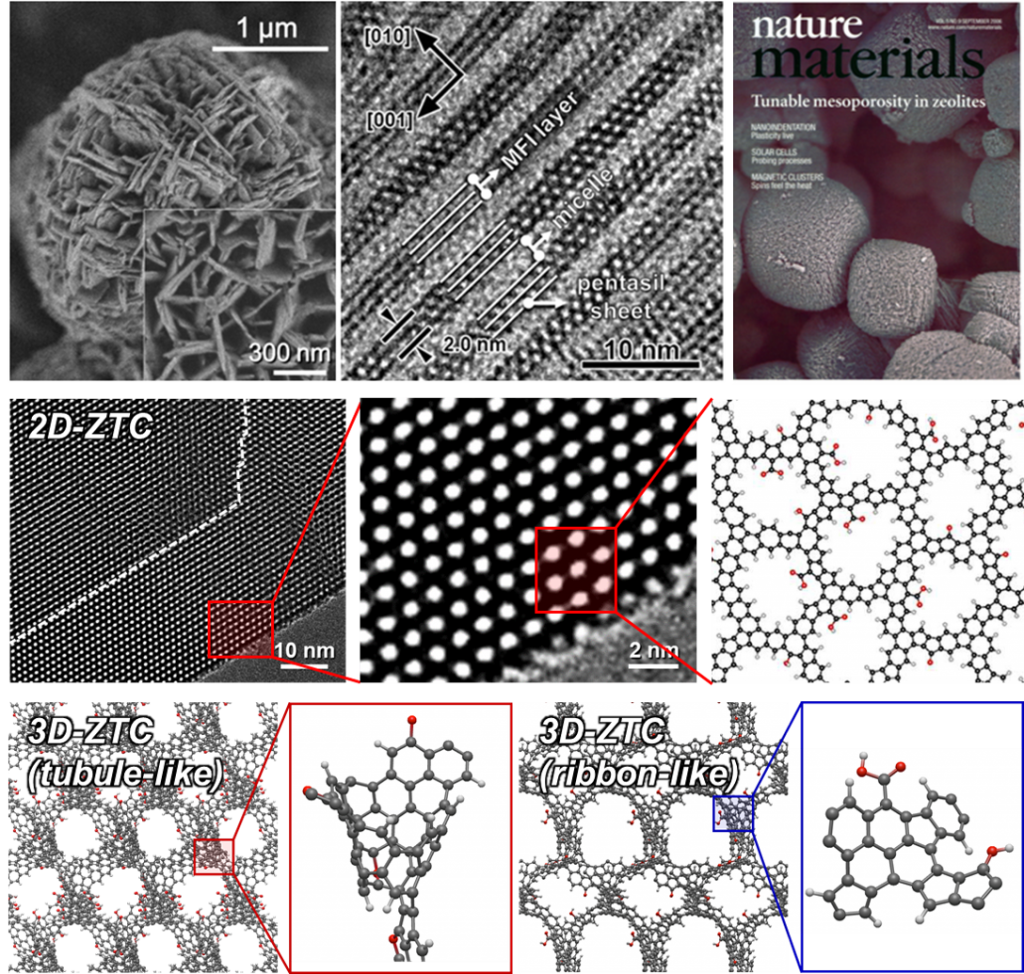
[1] “Amphiphilic Organosilane-Directed Synthesis of Crystalline Zeolite with Tunable Mesoporosity”
Nature Materials, 5, 718-723 (2006) – Link
[2] “Stable Single-Unit-Cell Nanosheets of Zeolite MFI as Active and Long-Lived Catalysts”
Nature, 461, 246-249 (2009) – Link
[3] “Bottom-up Synthesis of Two-Dimensional Carbon with Vertically Aligned Ordered Micropores for Ultrafast Nanofiltration”
Science Advances, 9, eade7871 (2023) – Link
[4] “Diversity in Atomic Structures of Zeolite-Templated Carbons and the Consequences for Macroscopic Properties”
JACS Au, 4, 1489-1499 (2024) – Link


